
CDC, FDA health warnings: Eyedrops may be linked to bacterial infections, one death
Yesterday, the U.S. Centers for Disease Control and Prevention (CDC) along with the U.S. Food and Drug Administration (FDA) both urged the public to immediately discontinue use of EzriCare Artificial Tears and Delsam Pharma Artificial Tears eyedrops, and said the eye drops could be linked to bacterial infections across the United States that have resulted in hospitalization, vision loss and one death.
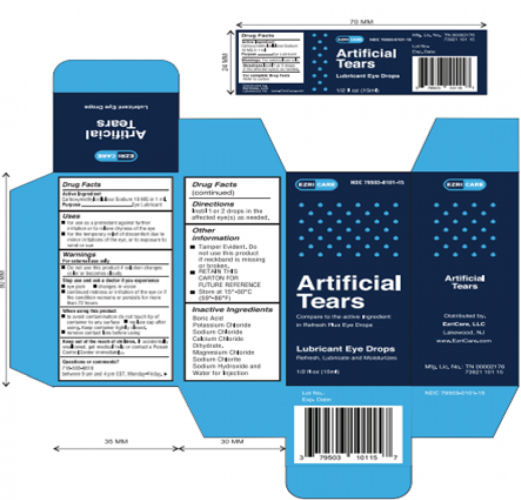
EzriCare brand artificial tear eye drops are often marketed in a blue package with light blue dots on the front and “EzriCare” printed on the upper left. Bottles of EzriCare eye drops often are blue with a light blue top.
The EzriCare artificial tears have been linked to at least 55 cases of bacterial infection in 12 states. Five of those people so far have had vision loss. One person died when the bacteria entered the bloodstream. ~ NBC News
As of January 31, 2023, a total of 55 patients in 12 states were identified with infections caused by a strain of the extensively drug-resistant Pseudomonas aeruginosa bacteria between May and January. Most patients had used artificial tears before the onset of their condition, and CDC and the FDA are investigating the infections.
The Food and Drug Administration said on Thursday night that the recall also included Delsam Pharma’s Artificial Tears, which are made by Global Pharma, the Indian company that manufactures the EzriCare eye drops. Global Pharma said that it was recalling the eye drops “out of an abundance of caution.” ~ New York Times
Patients reported more than 10 brands of artificial tears, and some patients used multiple brands. The majority of patients who used artificial tears reported using EzriCare Artificial Tears, a preservative-free product dispensed in multidose bottles. This was the only common artificial tears product identified across the four healthcare facility clusters.
Below are recommendations for the public:
- Discontinue using EzriCare Artificial Tears pending additional guidance from CDC and FDA.
- If patients were advised to use EzriCare Artificial Tears by their healthcare provider, they should follow up with their healthcare provider for an alternative artificial tears product to use.
- Patients who used EzriCare Artificial Tears and who have signs or symptoms of an eye infection, such as discharge from the eye, eye pain or discomfort, redness of the eye or eyelid, feeling of something in the eye, increased sensitivity to light, or blurry vision, should seek timely medical care. At this time, CDC does not recommend testing of patients who have used this product and who are not experiencing any signs or symptoms of infection.
Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops, distributed by /EzriCare, LLC- and Delsam Pharma, to the consumer level, due to possible contamination.
FDA Risk Statement: Use of contaminated artificial tears can result in the risk of eye infections that could result in blindness.
For more information on the health warnings, visit the CDC and FDA websites.
Also check out the video accompanying this article.
(Sources: CDC and FDA)
Written by Richard Webster, Ace News Today / Follow Richard on Facebook. Twitter & Instagram






