Ion-Pairing: A New Approach to Lyotropic Chromonic Liquid Crystal Assembly
Just today, researchers at Japan’s of Ritsumeikan University released word of a new study published in the journal Angewandte Chemie International Edition, describing their development of a new method for assembling lyotropic chromonic liquid crystals (LCLCs) using ion-pairing interactions. This breakthrough enables precise control of optical and electronic properties, paving the way for advanced applications in materials science, electronics, and display technologies.
This study showcases an innovative method for assembling lyotropic chromonic liquid crystals (LCLCs) through ion-pairing iπ–iπ interactions. Researchers successfully created highly ordered columnar structures by pairing oppositely charged π-electronic molecules in water. By varying applied magnetic fields and temperature, they were able to control the assembly’s order and subsequently, its optical properties. Additionally, this approach paves the way for designing specific molecular structures with advanced electronic applications.
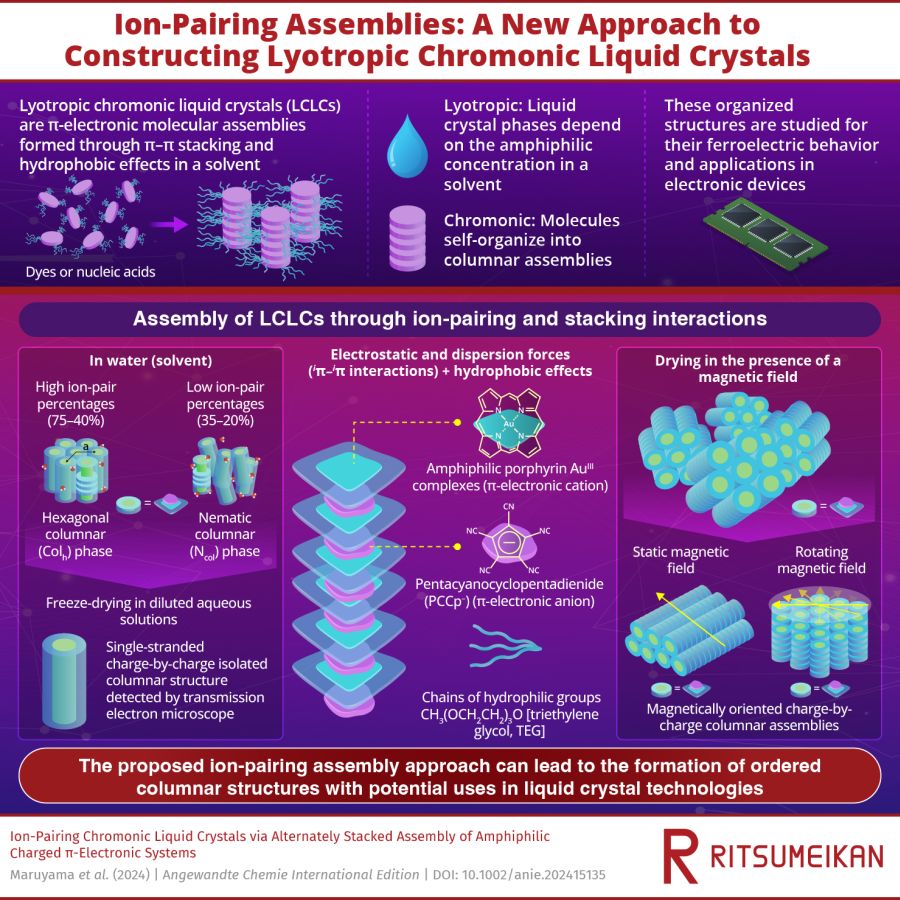
Self-assembling molecules into organized structures is highly valuable for developing new materials. One notable class of these materials is lyotropic chromonic liquid crystals (LCLCs), which are molecular assemblies of amphiphilic π-electronic molecules, with water-absorbing and water-repelling parts. The term “lyotropic” refers to liquid crystal phases that depend on the concentration of the molecules in a solvent, while “chromonic” indicates that these molecules are stacked into columnar assemblies. In a solvent, these structures are stabilized by π–π interactions and hydrophobic effects. Examples of such assemblies include the stabilization of nucleic acids in human bodies and the assembly of dye molecules.
Professor Hiromitsu Maeda, with his team at Ritsumeikan University, has been exploring new ways to build similar molecular assemblies. More than 15 years ago, they began investigating ion-pairing assemblies involving oppositely charged π-electronic systems, publishing their first findings on ion-pairing assemblies of porphyrin AuIII complexes in 2019.
In a recent study, published in the journal Angewandte Chemie International Edition, on September 23, 2024, a research team comprising Prof. Maeda, Dr. Koji Harano at National Institute for Materials Science, and Dr. Yasuhiro Ishida, at RIKEN, Japan, elucidated a novel method for assembling LCLCs, where the molecules are stabilized by electrostatic and dispersion forces known as iπ–iπ interactions, resulting in charge-by-charge columnar structures that can be oriented in the magnetic field.
“We started investigating the ion-pairing assemblies of charged π-electronic systems more than 15 years ago. The combination of the chemistry of π-electronic ion-pairing assemblies and that of amphiphilic assemblies inspired us to design amphiphilic charged π-electronic systems,” says Prof. Maeda.
The LCLC assembly was formed by pairing a positively charged porphyrin AuIII complex (1au+) with pentacyanocyclopentadienide (PCCp⁻) anion in water as the solvent. Hydrophilic triethylene glycol (TEG) chains were added to the complexes to stabilize the structure in water. The TEG units, composed of multiple ethylene glycol segments, form hydrogen bonds with water, helping to hydrate the complexes. The assembled molecules were studied using techniques such as synchrotron X-ray diffraction (XRD) and polarized optical microscopy (POM), which indicated the ordered arrangement of 1au⁺ stacked with PCCp⁻ anion via iπ–iπ interactions.
For the assembly of such molecules, it is important to choose the right ion pairs. The researchers also tested whether assemblies could be formed between positively charged porphyrin AuIII complexes and negatively charged chloride ions. However, in this configuration, the electrostatic repulsion between the positively charged 1au⁺ ions prevented the ions from coming together to form stable aggregates.
At high ion-pair concentrations (75–40%), the 1au⁺-PCCp⁻ ion pair formed highly ordered hexagonal columnar (Colh) structures. However, as the ion-pair concentration decreased, the structure became less ordered, transforming to a nematic columnar (Ncol) phase at concentrations around 35%. At very low ion-pair concentrations (below 15%), the structure dispersed completely, losing its liquid crystal properties. However, when freeze-dried, the single-stranded charge-by-charge assemblies were observed, indicating the charges of 1au⁺-PCCp⁻ were used for assembly via iπ–iπ interactions but not for hydration.
As molecules are arranged differently in each phase, their optical properties can vary depending on the phase formed. At low ion-pair concentrations, 1au⁺-PCCp⁻ ion pair loses their liquid crystal structure and becomes optically isotropic, interacting uniformly with light. In contrast, the ordered Colh and Ncol phases show optical anisotropy, reacting differently to light, based on the viewing angle. This enables the design of materials with specific optical properties.
The columnar structure of 1au⁺-PCCp⁻ exhibits anisotropic magnetic susceptibility, meaning it reacts differently to magnetic fields depending on the field’s orientation relative to its structure. Utilizing this property, the researchers successfully oriented the structure to achieve their desired arrangement. By drying 1au⁺-PCCp⁻ under the magnetic field, they achieved two distinct configurations: one, a horizontal orientation under a static magnetic field, and second, a vertical orientation under a rotating magnetic field.
Additionally, the orientation of the assemblies changed in response to temperature. At temperature of 60°C, the researchers noticed the Colh phase was formed from Ncol phase due to dehydration. This stimuli-responsive behavior of the material is highly advantageous, as it allows the creation of materials with desirable electronic and optical properties.
“The arrangement of π-electronic molecules is essential for producing new electronic materials and devices. Properties such as ferroelectricity can be controlled by the assembly modes of constituent molecules,” highlights Prof. Maeda. He further adds and concludes, “Our research has revealed new features of molecular assemblies by demonstrating the arrangement of charged π-electronic systems that resulted in further organized structures.”
***
Reference: Title of original paper: Ion-Pairing Chromonic Liquid Crystals via Alternately Stacked Assembly of Amphiphilic Charged π-Electronic Systems
Journal: Angewandte Chemie International Edition
***
About Ritsumeikan University, Japan: Ritsumeikan University is one of the most prestigious private universities in Japan. Its main campus is in Kyoto, where inspiring settings await researchers. With an unwavering objective to generate social symbiotic values and emergent talents, it aims to emerge as a next-generation research university. It will enhance researcher potential by providing support best suited to the needs of young and leading researchers, according to their career stage. Ritsumeikan University also endeavors to build a global research network as a “knowledge node” and disseminate achievements internationally, thereby contributing to the resolution of social/humanistic issues through interdisciplinary research and social implementation.
Website: http://en.ritsumei.ac.jp/
Ritsumeikan University Research Report: https://www.ritsumei.ac.jp/research/radiant/eng/
***
About Professor Hiromitsu Maeda from Ritsumeikan University, Japan: Dr. Hiromitsu Maeda is a professor at the Department of Applied Chemistry, College of Life Sciences, Ritsumeikan University and a Ritsumeikan Advanced Research Academy (RARA) Fellow. He completed his PhD from Kyoto University in 2004. Professor Maeda’s research interests include topics like physical organic chemistry, supramolecular chemistry, and materials science on π-electronic systems. Prof. Maeda has received several prizes, including the ChemComm Emerging Investigator Lectureship (2012) and Fellow of the RSC (2015), and has over 200 publications.
***
Posted by Richard Webster, Ace News Today
Follow Richard on Facebook, Twitter & Instagram






